PREDICTIX Clinical Study
Background
Major depressive disorder (MDD) is a common psychiatric disorder that is associated with a high burden on patients and their families. The World Health Organization estimates that, for every euro spent on digital solutions for mental health, countries can reap a return of four euros in improved health and productivity. Unfortunately, clinical trials indicate that current best-practice methods of determining the optimal treatment for a specific MDD patient lack efficiency. This inefficiency is plausibly caused by the polygenic nature and the phenotypic heterogeneity of MDD. Recent technological advancements lead to the accumulation of data through electronic health records, next-generation sequencing technologies, and sensory devices. This has paved the way to a new era of brain research; now we can start using Machine Learning (ML) as an advanced approach to understanding MDD.
There is an evident need for new approaches to help clinicians improve the treatment of depression and other psychiatric disorders. Applying machine learning methods to the accumulating data derived from Next Generation Sequencing (NGS) technologies, Electronic Health Records (EHR), and sensory devices can transform the way psychiatric disorders are treated.
Predictix Antidepressant
The Predictix Antidepressant tool was developed out of this growing need for personalized treatment selection for patients diagnosed as suffering from depression.
We aspired to create a prediction tool that relies on combinatorial data of clinical, demographic, and genetic information of each patient, in accordance with the applicable literature and guidelines.
We developed an algorithm, comprised of three main branches:
- Direct clinical guidelines for ADs
- Cytochromes recommendations
- Combinatorial ML models consisting of genetic, clinical, and demographic features

An algorithm output report, consisting of the likely effectiveness of 11 new-generation ADs, will then be produced in order to assist clinicians in applying the most informed and suitable treatment for each patient.
One challenge in designing any prediction algorithm is in selecting the right combination of features that will predict a well-defined clinical outcome. The PREDICTIX algorithm and pipeline may be used as a tool to tackle this challenge and act as a decision-supporting tool for clinicians, generating more precise, efficient, quick, and cost-effective treatment for depression.
Taliaz is currently in the start-up stage of the study in which regulatory submissions are taking place in parallel to other administrative tasks.
Patient recruitment is expected to begin by August 2021 with the aim to enrol up to 354 eligible patients.

The Predictix project has received funding from the European Union’s research and innovation program under grant agreement No: 874082
The Predictix Clinical Study, in-depth
A Prospective, Randomized, Double-Blind (Subject and Rater) Controlled Study to Confirm the Effectiveness of Predictix Genetics Antidepressant -Guided Treatment in Adults With Major Depressive Disorder (MDD)
The study’s key objectives are as follows:
Primary objective:
- To confirm the effectiveness of the Predictix Genetics Antidepressant software tool to improve antidepressant treatment response in patients with MDD.
Secondary objectives:
- To confirm the effectiveness of the Predictix Genetics Antidepressant software tool to improve antidepressant treatment remission rates in patients with MDD.
- To retrospectively evaluate the effectiveness of a Predictix software based on a patients’, clinical, demographic and/or behavioral inputs only (without the use of the genetics input).
Safety objective:
- To confirm the safety of the Predictix Genetics Antidepressant software.
Exploratory objective:
- To evaluate the effect of the Predictix Genetics Antidepressant software use on patients’ care outcomes in terms of economic burden and social impact.
- To evaluate the device usability via questionnaires completed by the treating physician and the patient.
Study Cohorts and Arms:
Treatment as Usual (TAU) and Predictix Guided Treatment (PGT).
The study will compare the rate of treatment response and remission among both groups; TAU vs PGT.
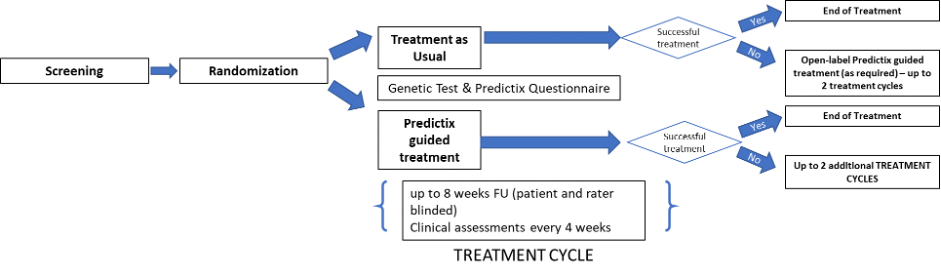
Primary Endpoint:
Response rate at the completion of the first treatment cycle. Response is defined as a reduction from baseline of at least 50% (≥50%) in MADRS.
Secondary Endpoints:
- Remission rate at the completion of the first treatment cycle. Remission is defined as a MADRS < 10.
- Change from baseline in MADRS over time.
- Time to response.
- CGI-S score over time.
- CGI-I score over time.
- Prediction rates of the Predictix Genetics Antidepressant software based on a patients’, clinical, demographic and behavioral inputs only (without the use of the genetics input).
- Overall PGT Response rate, defined as response rate at the completion of the first treatment cycle for subjects randomized to PGT, and at the eighth week during the open-label phase for subjects randomized to TAU.
- Overall PGT Remission rate, defined as remission rate at the completion of the treatment cycles for subjects randomized to PGT, and at the eighth week during the open-label phase for subjects randomized to TAU.
Safety Endpoints:
- Safety endpoints include Adverse Events (AE) and Serious AE (SAE)
Exploratory Endpoints:
- Economic burden and social impact on patients, employers, health system and payers:
Parameters analyzed from the patient’ electronic medical data record retrospectively (6 months prior to enrollment) and following a long-term FU period (6 months and 12 months post enrollment):
- Attendances and contacts to healthcare providers (in ambulatory, total number, average duration and type of physician visit). This should include both basic and supplementary insurance (GPs and any other specialists, including neurologists, psychiatrists, GEs etc.)
- Diagnostic and imaging tests – number of test and reason taken
- Visits to emergency departments – number and reason
- Hospital admissions – duration, type (acute vs long-term), primary/secondary reason for admission
- Number of antidepressant medications used –duration, dosage and reason
- Number of other medications used –duration, dosage and reason
- Medical procedures – number, type and reason
- Visits to other care providers excluding doctors (social workers, psychologists etc.) – total number, average duration and type of care provider. This should include records from both basic and supplementary insurance.
- Work Productivity and Activity Impairment (WPAI) questionnaire.
- EQ-5D-5L Questionnaire
- Medication Change – Number of subjects who changed their baseline antidepressant medication regimens.
- Medication Choice – Proportion of time that the psychiatrist prescribed a medication that was recommended by the Predictix.
- FIBSR and PRISE questionnaires
Usability and satisfaction of the Predictix software tool –questionnaires to be completed by the treating physician.
Study Population:
The study population will consist of male and female subjects aged 18 to 75, diagnosed with MDD:
- experiencing a major depressive episode backed by diagnostic criteria for MDD for which an initial antidepressant is indicated.
Inclusion Criteria:
- Male or female at the age of 18-75 years old at time of screening.
- Primary diagnosis of Major Depressive Disorder (without psychosis) based on DSM-5 criteria and MINI 7.0.
- MADRS score ≥22
- No other causes of depressive symptoms other than MDD.
- Ability to read, understand and sign an informed consent document.
- Not more than 2 past failed pharmacologic interventions for the current depressive episode.
- If subject is female and at reproductive age, she must be tested negative for pregnancy.
- If subject is female and at reproductive age with childbearing potential (i.e., not post-menopausal or surgically sterilized) she must agree to use adequate birth control methods during the whole study duration.
Exclusion Criteria:
- Patient is diagnosed with other major psychopathologies (i.e. schizophrenia, bipolar disorder, psychotic depression, geriatric depression).
- Patient requires antipsychotic medication or mood stabilizers (other than study medication), lithium, carbamazepine, valproate and other that may have an antidepressant effect.
- Electroconvulsive therapy (ECT) or transcranial magnetic stimulation therapy (TMS) conducted in the past or started within 90 days of screening or planned during the study.
- Nonpsychopharmacologic drugs with CNS effects that have been taken for less than 30 days prior to baseline.
- Subjects with a vagus nerve or deep brain stimulator.
- Patient is at substantial suicidal risk as determined by the Mini Neuropsychiatric Interview (MINI) Suicidality subscale for suicide attempts and/or judged by the treating physician.
- Patient has any current unstable medical condition or surgical illness.
- Patient has history of seizure or convulsions.
- A current status of dependence to a drug or alcohol.
- Inadequate communication with the patient.
- Patient has participated in another clinical study in the last 30 days preceding this study.
- In the investigator’s judgement, patient is not able to provide written informed consent and follow protocol requirements.
- Pregnant women.
